How to Calculate Empirical Formula
Count the number of moles of each type. Divide molar mass of BH 3 with the empirical formula mass.

Calculating Empirical Formula Mcat Study Chemistry Labs Calculus
You can use the empirical formula to find the molecular formula if you know the molar mass of the compound.

. Find the number of moles of each element in the compound by dividing the mass in grams of each with its atomic mass. Scroll down the page for more examples and solutions. You have the option of using mass data in grams or percent composition.
This video goes into detailed steps on how to find the empirical formula of a compound. Calculate empirical formula mass EFM EFM of BH 3 1384gmol. Hooray for no more confusionCheck out my NEW complete guide on Empir.
To calculate the formula of a compound. You can use information about reacting masses. The molecular formula is.
Find the simplest formula. Using Empirical Formula to Find Molecular Formula. We can determine the empirical formula by using the proportion of each element in the compound data.
Here is an example. Calculate the mass of each element in grams. The empirical formula of a substance can be calculated from the experimentally determined percent composition the percentage of each element present in a pure.
How to calculate the Empirical Formula from Element Proportions. Empirical Formula the simplest whole. For example the empirical formula of a hydrocarbon is CH 2 and its M r is 42.
Molar mass EFM 277 gmol 1384gmol. The following diagram gives the steps to calculate the empirical formula when given the mass percentages. Since the total mass of the final product was 0378 we find.
So the simplest formula of the compound is CH2O. 1 In any empirical formula problem you must first find the mass of the elements in the compound. 32 16 16 64.
The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound. 7 Use the scaling factor computed just above to determine the molecular formula. Divide each value by the lowest figure.
SO 2 times 1 gives SO 2 for. 6 Divide the molecule weight by the EFW 6407 64 1. Calculate the empirical formula.
Convert numbers to whole numbers by multiplying by the smallest number that can make the decimals whole numbers. The steps for determining a compounds empirical formula are as follows. Suppose you have a compound of aluminum oxide if the.
To calculate the empirical formula first determine the relative masses of the elements present. Well do some empirical formula calculations and problems in just a second but first lets get some definitions out of the way. Also our online empirical formula.
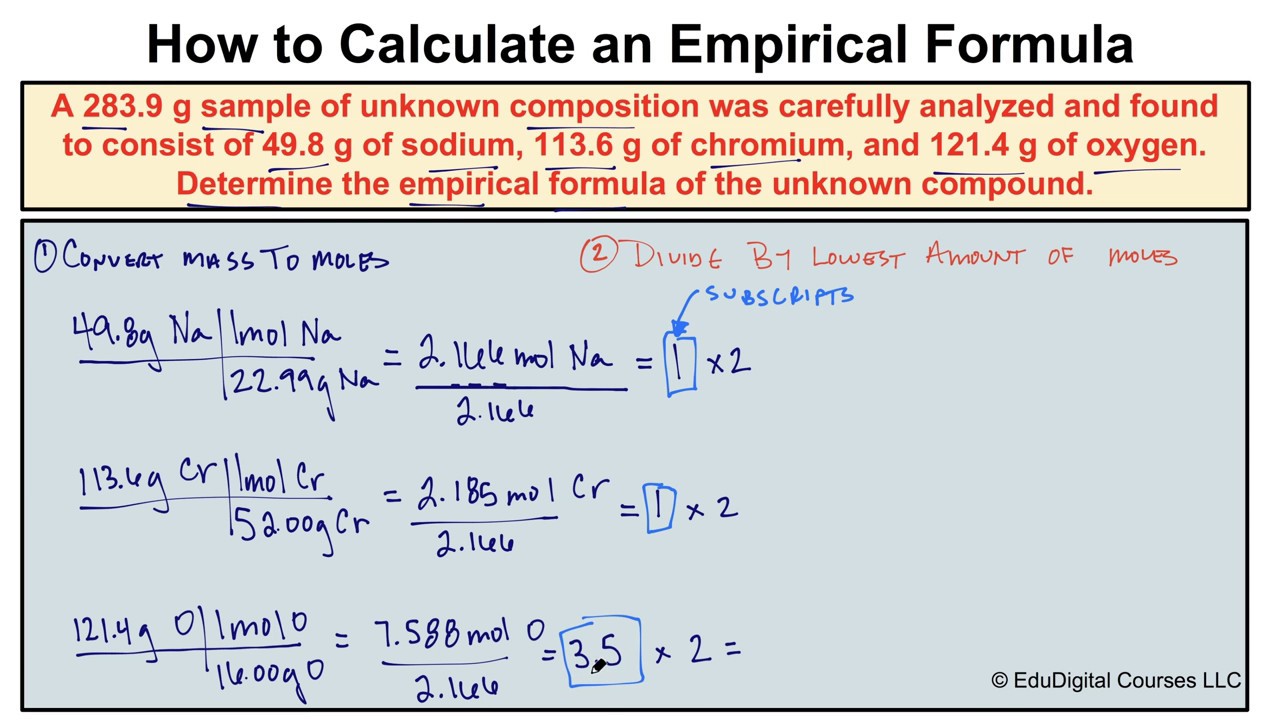
How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Scientific Method Worksheet

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Calculating Empirical Formula Chemistry Math Oxygen

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
No comments for "How to Calculate Empirical Formula"
Post a Comment